Looking to create a mesmerizing chemical reaction? Look no further than learning how to make devil’s toothpaste step by step. This exhilarating experiment involves a few simple ingredients and promises a visually stunning outcome. Get ready to harness the power of chemistry and unleash your inner scientist as we dive into the exciting world of devil’s toothpaste!
How to Make Devil’s Toothpaste Step by Step
Welcome, young scientists! Today, we are going to embark on an exciting experiment to create something called devil’s toothpaste. It may sound mysterious and magical, but it’s actually a thrilling science project that involves chemistry and reactions. Get ready to put on your safety goggles and dive into the world of foamy explosions!
Materials You’ll Need
Before we start creating devil’s toothpaste, let’s gather all the necessary materials. Here’s what you’ll need:
- A plastic bottle
- Hydrogen peroxide (6% concentration)
- Dish soap
- Dry yeast
- Warm water
- Food coloring (optional)
- Safety goggles
- Measuring cups and spoons
- A tray or container for the experiment
Make sure to ask an adult for help with handling some of these materials, especially the hydrogen peroxide.
Setting Up the Experiment
Now that we have all our materials ready, it’s time to set up our devil’s toothpaste experiment. Follow these steps carefully:
- Put on your safety goggles to protect your eyes.
- Place the plastic bottle on the tray or container to catch any spills.
- Pour ½ cup of hydrogen peroxide into the bottle.
- Add a squirt of dish soap into the bottle. This will help make the foam more stable.
- If you want colorful toothpaste, add a few drops of food coloring into the bottle.
- In a separate cup, mix 1 tablespoon of dry yeast with 3 tablespoons of warm water. Stir well until the yeast is completely dissolved.
- Quickly pour the yeast mixture into the bottle with the hydrogen peroxide and dish soap.
The Reaction Begins
As soon as the yeast mixture touches the hydrogen peroxide, a chemical reaction will begin. The yeast acts as a catalyst, speeding up the decomposition of hydrogen peroxide into water and oxygen gas. This reaction releases oxygen gas in the form of bubbles, creating the foamy toothpaste effect.
Now, step back and watch in amazement as your devil’s toothpaste starts to foam and grow out of the bottle. The foam may overflow from the bottle, so make sure to do this experiment in a safe and easy-to-clean area.
Understanding the Science Behind It
Devil’s toothpaste is a fun demonstration of an exothermic reaction, which means it releases heat as it occurs. The decomposition of hydrogen peroxide is an exothermic reaction that generates oxygen gas and water. The dish soap helps trap the oxygen gas, creating the foamy toothpaste effect.
By adding yeast to the hydrogen peroxide, we speed up the decomposition process, causing the rapid release of oxygen gas and creating the foam that looks like toothpaste. It’s important to note that the reaction is safe as long as you handle the materials properly and follow the steps correctly.
Experiment Variations
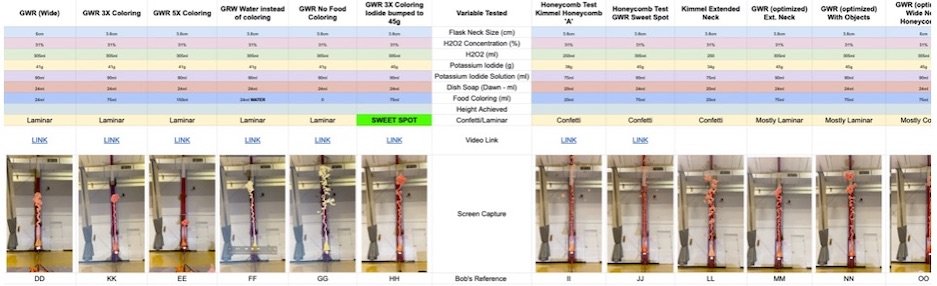
Once you’ve mastered the basic devil’s toothpaste experiment, you can try some variations to see how they affect the reaction. Here are a few ideas to explore:
Vary the Concentration of Hydrogen Peroxide
Try using different concentrations of hydrogen peroxide, such as 3% or 9%, and observe how it affects the foaming reaction. Remember to adjust the amount of yeast accordingly to maintain a safe and exciting reaction.
Change the Temperature of the Water
Experiment with using cold or hot water to mix with the yeast and observe how the reaction rate changes. Warmer water may speed up the reaction, leading to a more vigorous foaming action.
Explore Different Catalysts
Instead of yeast, try using other catalysts like potassium iodide or manganese dioxide to see how they influence the decomposition of hydrogen peroxide. Each catalyst may produce a unique foaming effect.
Congratulations, young scientists! You’ve successfully created devil’s toothpaste through a fascinating chemical reaction. Remember to clean up your experiment area and dispose of the materials safely. Science is all about exploration and discovery, so keep experimenting and learning about the amazing world of chemistry!
Stay curious, stay safe, and keep on creating wonders with science!
how to make DEVILS toothpaste #experiment #home #science #sugar #sulphuricacid #cool #chemistry #the
Frequently Asked Questions
What ingredients are needed to make devil’s toothpaste?
To make devil’s toothpaste, you will need hydrogen peroxide, dish soap, food coloring, and a packet of dry yeast.
How do you mix the ingredients to create devil’s toothpaste?
First, mix the hydrogen peroxide and dish soap in a container. Then, add food coloring for a desired color. In a separate small container, mix warm water with dry yeast until it dissolves. Finally, pour the yeast mixture into the hydrogen peroxide mixture and watch the foam explosion!
What safety precautions should be taken when making devil’s toothpaste?
When working with hydrogen peroxide, always wear gloves and safety goggles to protect your skin and eyes. It’s also important to perform this experiment in a well-ventilated area to avoid inhaling any fumes.
Final Thoughts
In conclusion, making devil’s toothpaste is a simple and exciting experiment that can be done with common household items. To make devil’s toothpaste step by step, begin by mixing hydrogen peroxide, dish soap, and food coloring in a plastic bottle. Then, add a packet of yeast and watch as the foam erupts. Finally, enjoy the vibrant eruption created by this fun and educational science project. Experiment safely and have fun with making devil’s toothpaste step by step!





+ There are no comments
Add yours